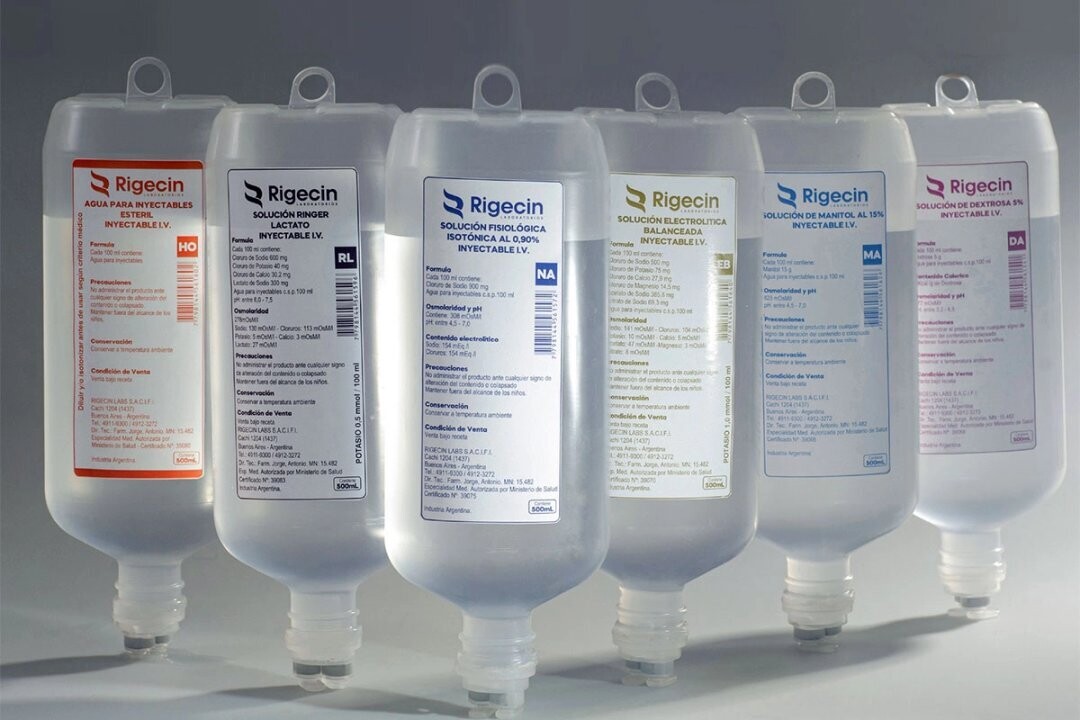
The National Administration of Medicines, Food and Medical Technology (ANMAT) has ordered the ban on the use, distribution, and sale nationwide of two batches of "Isotonic Physiological Solution Rigecin 0.90%", produced by Rigecin Labs S.A., due to a critical quality deviation with a high impact on health.
The order affects batches NA5187 S2 (expiration 01/2027) and NA5675 S1 (expiration 04/2027), which had unauthorized and non-compliant packaging, thus failing to meet the required standards for large-volume parenteral solutions.
ANMAT also ordered the mandatory recovery of the market for the affected products and the opening of a health summary against the company for alleged violations of Law 16.463 and various regulatory provisions.
Health authorities nationwide and in the City of Buenos Aires were instructed to ensure compliance with the ban and the product recall.
The measure was adopted preventively and as a priority, following evaluations that determined a serious risk to public health from the use of the affected batches.